
Protein analysis
HCP analysis
Innovative Technology Support Group for Life Science and Drug Discovery
HCP analysis (Host cell protein)
Test Introduction
The production of biomedicine uses the same subculture, but contamination occurs as the protein inside the host cell is refined simultaneously with the medicine during the production process. Because the residual host cell protein (HCP) negatively affects the drug stability, it is essential to detect and the 동정 HCP contamination during the biopharmaceutical development process. In order to supplement the ELISA-based HCP analysis, the orthogonal detection and monitoring technologies including LC-MS/MS and CESI-MS/MS are used. For instance, LC-MS/MS not only detects and performs the characteristic analysis of the HCP inside the substance used for biopharmaceutical production, but also provides the direct import of the individual protein equivalency information from the entire data instead of the total HCA, which is the limitation of the current ELISA-based analysis method.
Sample
- Antibody
- Antibody-drug conjugates
- Recombinant protein
- Toxine
- Vaccine
Analysis Process
- Sample pre-treatment
- LC-MS/MS
- Data Analysis
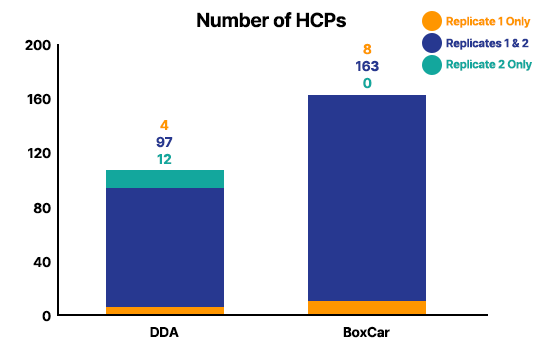
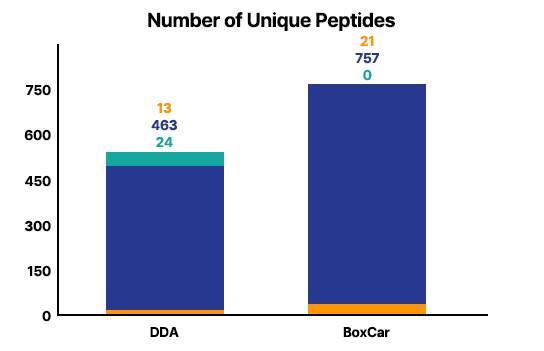
Analysis Result
| Accession | Description | Coverage [%] | # Peptides | # PSMs | # Unique Peptides | MW [kDa] | Abundance (Area) | Area % |
|---|---|---|---|---|---|---|---|---|
| API protein | 98 | 27 | 300 | 21 | 22.2 | 10,267,645,316 | 97.61 | |
| P54375 | API protein II | 93 | 17 | 100 | 11 | 22.5 | 210,558,111 | 2 |
| P80879 | General stress protein | 48 | 6 | 9 | 6 | 16.6 | 9,396,655 | 0.09 |
| P40767 | DL-endopeptidase CwlO | 11 | 6 | 9 | 6 | 51 | 12,968,304 | 0.12 |
| O34919 | nucleotidohydrolase YosS | 36 | 5 | 7 | 5 | 16.1 | 6,362,273 | 0.06 |
| O31567 | Sderophore-binding lipoprotein | 28 | 5 | 6 | 5 | 36.3 | 4,796,955 | 0.05 |
| P80643 | carrier protein | 31 | 2 | 3 | 2 | 8.6 | 4,171,635 | 0.04 |
| O34866 | carboxypeptidase | 11 | 2 | 2 | 2 | 30.8 | 1,258,550 | 0.01 |
| P02394 | 50S ribosomal protein | 18 | 2 | 2 | 2 | 12.7 | 1,666,073 | 0.02 |
- Download Your Request
 Analysis Consulting : 042-384-0702, 070-5014-5710
Analysis Consulting : 042-384-0702, 070-5014-5710