mRNA analysis
mRNA Analysis
Inovative Technology Support Group for Life Science and Drug discovery
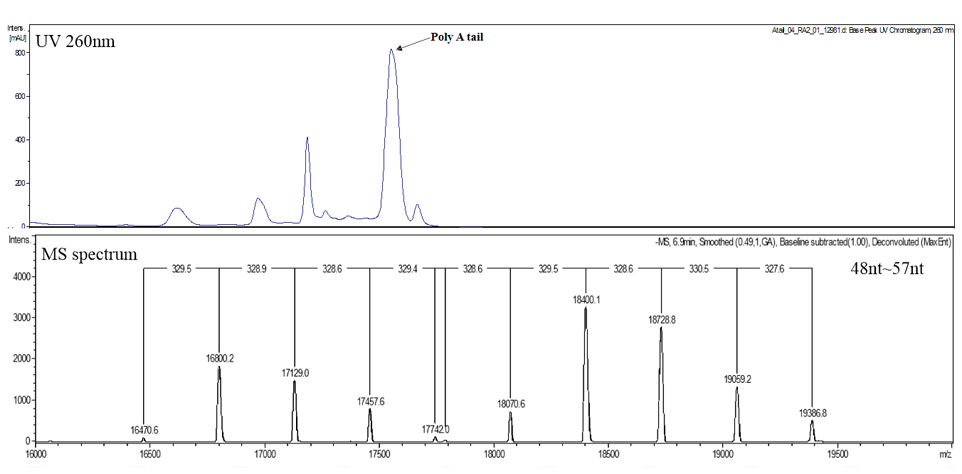
3’ Poly A analysis
Test Introduction
mRNA vaccines have shown impressive safety and efficacy in clinical trials, outperforming vaccines based on alternative technologies. As mRNA vaccines are considered gene therapies, FDA guidance requires extensive characterization of product-related impurities. These may include populations of mRNA molecules with slight errors in their sequence, known as sequence variants.
Sample
- Raw mRNA pharmaceuticals
- Final mRNA pharmaceuticals
Analysis Process
- mRNA
- RNase T1 cleavage
- Poly A analysis by LC-MS
Analysis Result