
Protein analysis
Physicochemical Properties
Innovative Technology Support Group for Life Science and Drug Discovery
Capillary Electrophoresis : CE-SDS (capillary electrophoresis sodium dodecyl sulfate)
Test Introduction
The mutation of a small amount of impurities in biopharmaceuticals is a common issue that occurs within the overall biomedicine pipeline, which would result in negative impacts in efficacy and stability. Thus, it is important to keep pace with the procedure through minimizing the time required for the pretreatment and processing of the sample. Making right decisions is possible based on the consistent quantitative analysis results from the CE-SDS technology that provides raw data with high reproducibility for obtaining approval by a regulatory authority.
Sample
- Antibody
- Antibody-drug conjugates
- Recombinant protein
- Toxine
- Vaccine
Analysis Process
- Sample pre-treatment
- CE-SDS
- M.W ID & Quantitative
analysis
Analysis Result
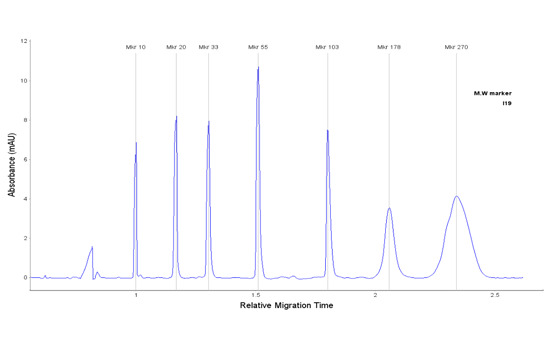
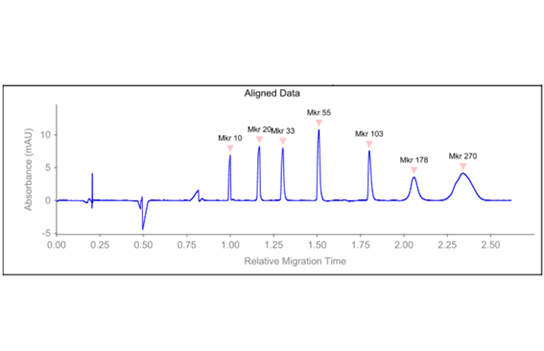
Peaks
좌우로 스크롤하여 내용을 확인하세요.
| Peak | Name | Time | RMT | MW(kDa) | Height | Raw Area | Area | %Total | %Area | Width | S/N | Baseline | Resolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mkr 10 | 802.7 | 1.000 | 10 | 6.9 | 115.4 | 144.1 | 8.5 | 622.8 | 6.2 | |||
| 2 | Mkr 20 | 938.0 | 1.169 | 20 | 8.2 | 187.1 | 200.1 | 15.1 | 11.1 | 743.3 | 7.8 | 7.55 | |
| 3 | Mkr 33 | 1046.2 | 1.303 | 33 | 8.0 | 197.2 | 188.6 | 14.3 | 11.6 | 722.4 | 7.9 | 6.48 | |
| 4 | Mkr 55 | 1212.3 | 1.510 | 55 | 10.8 | 269.8 | 222.5 | 16.8 | 11.8 | 973.8 | 10.8 | 8.84 | |
| 5 | Mkr 103 | 1444.8 | 1.800 | 103 | 7.6 | 224.0 | 154.6 | 11.7 | 13.3 | 682.2 | 6.8 | 14.44 | |
| 6 | Mkr 178 | 1651.8 | 2.058 | 178 | 3.6 | 277.0 | 167.5 | 12.7 | 33.9 | 321.3 | 3.5 | 4.85 | |
| 7 | Mkr 270 | 1877.8 | 2.340 | 270 | 4.2 | 733.2 | 389.7 | 29.5 | 87.6 | 375.0 | 4.1 | 2.30 |
- Download Your Request
 Analysis Consulting : 042-384-0702, 070-5014-5710
Analysis Consulting : 042-384-0702, 070-5014-5710